Pyrrole-based macrocycles, mechanically interlocked molecules and cages: dynamics, tautomerism, chirality
Bartosz Szyszko
Faculty of Chemistry, University of Wrocław, ul. F. Joliot-Curie 14, 50-383 Wrocław, Poland
bartosz.szyszko@uwr.edu.pl, www.bszyszko.pl
Mechanically interlocked molecules (MIMs) represent a unique class of supramolecular systems characterized by the presence of a mechanical bond, with rotaxanes and catenanes serving as prototypical examples.1 This talk will focus on two fundamental aspects of mechanically interlocked pyrrole-based MIMs. First, it will explore the use of the diiminopyrrole motif in subcomponent self-assembly,2-4 to construct molecular knots and links that exhibit intramolecular dynamics facilitated by the fluxionality of coordinated metal centers. Furthermore, the discussion will highlight how the introduction of more complex pyrrole-derived building blocks enables the synthesis of mechanically interlocked porphyrinoids, offering new avenues for functional molecular design. The exploitation of iminopyrrole synthons for building intriguing cage architectures demonstrating unique coordination plasticity and tautomeris will be demonstrated.5-7
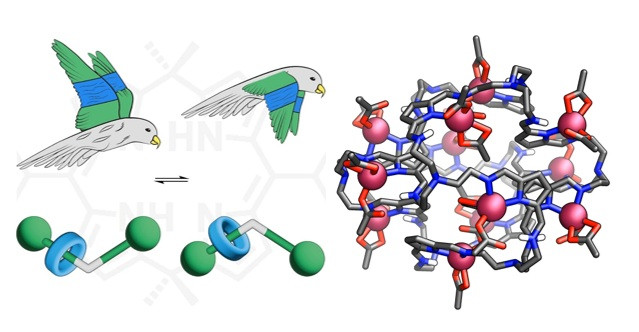
The second part of the talk will discuss a new class of rotaxanes incorporating dipyrromethane stoppers designed to construct dynamic MIMs.8 A key focus will be on the discovery and characterization of a new mode of molecular motion, referred to as fluttering, which is reminiscent of butterfly wing flapping. The multimodal motion can be precisely modulated using simple acid-base chemistry, providing a versatile platform for designing stimuli-responsive molecules. The higher-order calix[4]phyrin-based mechanically interlocked molecules will also be discussed, further expanding the structural and functional diversity of these systems.9 Eventually, it will be demonstrated how the molecular editing of an achiral [2]rotaxane comprising a pyrrole ring allows it to transform into the mechanically planar chiral species.10
References
[1] Bruns, C. J.; Stoddart, J. F. The Nature of the Mechanical Bond: From Molecules to Machines, 1st ed.; Wiley, 2016.
[2] Matviyishyn, M., Białońska, A., Szyszko, B.¸ Angew. Chem. Int. Ed. 2022, anie.202211671.
[3] Sarwa, A., Białońska, A., Sobieraj, M., Martínez, J. P., Trzaskowski, B., Szyszko, B. Angew. Chem. Int. Ed. 2024, 63, e202316489.
[4] Sarwa, A., Khmara, A., Konieczny, K. A., Kulesza, D., Zych, E., Trzaskowski, B., Szyszko, B., Angew. Chem. Int. Ed., 2025, e202423962.
[5] Sukiennik, J., Sarwa, A., Perdek, J., Siczek, M., Szyszko, B., 2025, Chem. Eur. J. 2025, DOI: 10.1002/chem.202502714.
[6] Sarwa, A. Białońska, A., Garbicz, M., Szyszko, B. Chem. Eur. J. 2023, e202203850.
[7] Trzaskowski, B., Martínez, J., Sarwa, A., Szyszko, B., Goddard III, W. J. Phys. Chem. A2024, 128, 3339-3350.
[8] Grzelczak, R. A., Basak, T., Trzaskowski, B., Kinzhybalo, V., Szyszko, B., Angew. Chem. Int. Ed. 2025, e202413579.
[9] Grzelczak, R. A., Perdek, J. P., Siczek, M., Chmielewski, P. J., Szyszko, B. Org. Lett.2025, 27, 6310–6315.
[10] Grzelczak, R. A., Perdek, J. P., Siczek, M., Chmielewski, P. J., Szyszko, B., submitted, 2025
